
By myhandsanitizershop September 30, 2025
Hand sanitizers have become an essential part of everyday life, especially in the wake of global health crises such as the COVID-19 pandemic. From schools and workplaces to retail stores and hospitals, these products are used to prevent the spread of harmful germs and keep people safe.
However, despite their widespread use, mislabeling issues in hand sanitizers have emerged as a significant concern. Mislabeling not only misleads consumers but also puts public health at risk by creating a false sense of security about the effectiveness and safety of these products.
This article provides a comprehensive, detailed guide on common mislabeling issues in hand sanitizers, why they occur, how to identify them, and what consumers, manufacturers, and regulators can do to address them.
By the end, readers will understand the critical importance of accurate labeling, the risks associated with misrepresentation, and practical ways to stay informed and protected.
Why Accurate Labeling Matters in Hand Sanitizers
Labels on hand sanitizers are more than just decorative packaging—they serve as vital communication tools between manufacturers and consumers.
Labels provide information about the product’s active ingredients, percentage of alcohol, usage instructions, warnings, expiration date, and certifications. When labeling is accurate, consumers can make informed choices and use the product effectively and safely.
Accurate labeling matters for several reasons:
- Health and Safety: The primary reason people use hand sanitizers is to protect themselves from harmful bacteria and viruses.
If the product is mislabeled—such as overstating its alcohol content or omitting harmful ingredients—it may not provide the promised protection, leaving consumers vulnerable. - Compliance with Regulations: In most countries, hand sanitizers are regulated by health authorities such as the FDA (Food and Drug Administration) in the United States or European Medicines Agency (EMA) in Europe.
These agencies set clear standards for labeling requirements. Mislabeling is a violation of these standards and may result in recalls, penalties, or even product bans. - Consumer Trust: Mislabeling erodes consumer confidence. Once a company is caught misrepresenting its product, regaining consumer trust can be extremely difficult.
In a competitive market, accurate labeling becomes not only a legal requirement but also a business necessity. - Public Health Implications: Inaccurate labeling on a widely used product such as hand sanitizer can have ripple effects across communities.
For example, a mislabeled sanitizer with insufficient alcohol may contribute to the spread of infectious diseases, especially in high-traffic environments like schools or public transport.
Thus, label accuracy is both a regulatory requirement and an ethical responsibility. Yet, despite strict standards, many mislabeling issues persist in the marketplace.
Common Types of Mislabeling in Hand Sanitizers
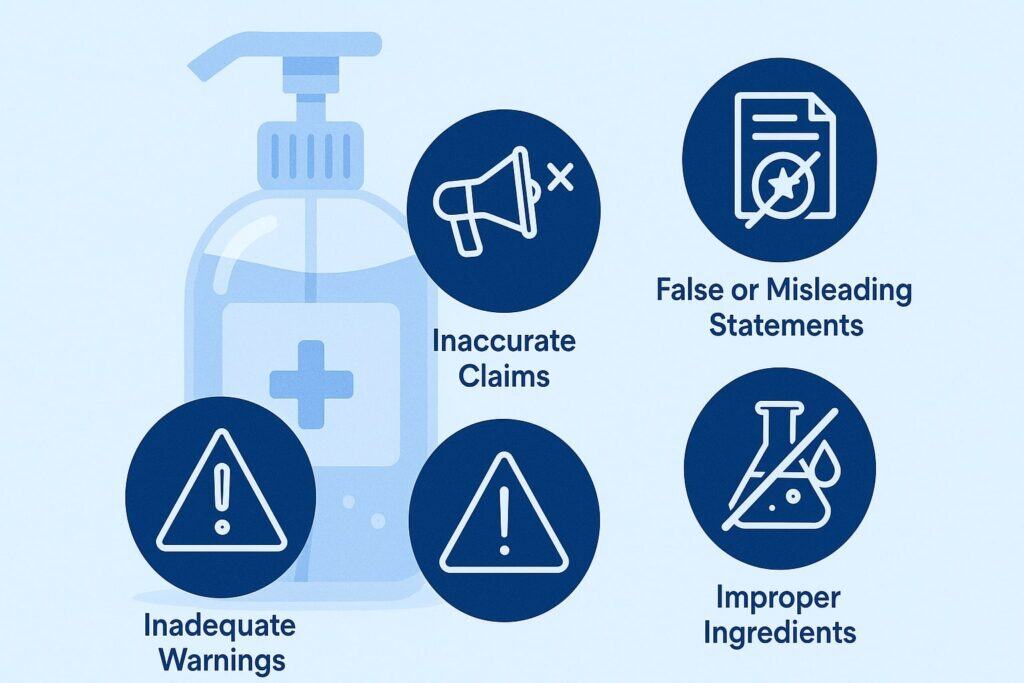
Mislabeling can take many forms, and each one poses unique risks. Below are some of the most common issues found in hand sanitizer products:
1. Incorrect Alcohol Content
Perhaps the most frequent mislabeling issue is overstating or understating alcohol content. Effective sanitizers should contain at least 60% ethanol or 70% isopropyl alcohol to kill germs effectively.
However, some products falsely claim to meet this threshold when, in reality, their alcohol concentration is lower. This makes them ineffective against viruses and bacteria, misleading consumers into a false sense of safety.
2. Hidden or Dangerous Ingredients
Some products have been found to contain methanol, a toxic substance that can cause blindness, organ failure, or even death if ingested or absorbed in significant quantities.
Many such products were recalled worldwide after being mislabeled as safe alternatives. Consumers often discover these risks only after regulatory agencies issue warnings.
3. Misleading Claims of Effectiveness
Some sanitizers claim to kill “99.99% of germs” without adequate scientific evidence. Others exaggerate their effectiveness against specific viruses such as COVID-19, even when no clinical testing supports those claims.
Such false marketing tactics create unrealistic expectations and may lead to improper reliance on hand sanitizers instead of comprehensive hygiene practices like handwashing.
4. Missing or Incomplete Warnings
Labels should clearly warn consumers about potential risks, such as flammability or keeping the product away from children. Many mislabeled sanitizers either minimize or completely omit these warnings, creating hazardous situations.
For example, leaving out flammability warnings can lead to accidental fires when sanitizers are used near open flames.
5. Misrepresentation of Certification or Approval
Some hand sanitizers falsely display logos of recognized regulatory bodies or certifications like “FDA approved.” In reality, the FDA does not “approve” over-the-counter sanitizers—it only monitors compliance.
Such misrepresentation misleads consumers into believing the product has a higher standard of safety and efficacy.
6. Expired or Unclear Expiration Dates
Another frequent issue is the absence of expiration dates or misleading information about product shelf life. Alcohol in sanitizers can evaporate over time, reducing effectiveness. Without accurate expiration labeling, consumers may unknowingly use ineffective products.
7. False “Natural” or “Organic” Claims
Marketing strategies often misuse terms like “natural,” “organic,” or “chemical-free” to attract eco-conscious consumers. However, such claims are often not verified or substantiated.
Inaccurate “greenwashing” misleads buyers into thinking they are purchasing safer or more sustainable options when they are not.
Each of these mislabeling issues demonstrates how errors—or intentional misrepresentation—can endanger public safety, violate laws, and mislead consumers.
Regulatory Oversight and Industry Standards
Governmental bodies and industry organizations play an essential role in preventing and addressing mislabeling. In the United States, the FDA regulates hand sanitizers as over-the-counter (OTC) drugs. This means manufacturers must follow strict guidelines for:
- Active ingredient disclosure
- Alcohol content minimums
- Label formatting and warnings
- Testing and quality assurance
Globally, organizations such as the World Health Organization (WHO) provide recommended formulations and safety standards. Meanwhile, countries like Canada (through Health Canada) and regions like the EU enforce their own regulations.
During the pandemic, however, the surge in demand led to a flood of new products entering the market—some from unregulated manufacturers. Regulatory agencies issued dozens of recalls after discovering widespread mislabeling, particularly related to methanol contamination.
To ensure compliance, regulators conduct random testing, inspections, and mandatory recalls. However, limited resources and the sheer volume of products mean some mislabeled items still slip through. This underscores the importance of consumer awareness and vigilance.
Impact of Mislabeling on Consumers and Public Health

Mislabeling hand sanitizers has consequences that extend beyond simple inconvenience. It has direct and indirect impacts on both individual users and broader communities:
- Health Risks: Using a mislabeled sanitizer with insufficient alcohol content may leave individuals exposed to pathogens. Worse, products containing harmful ingredients such as methanol pose serious toxicological risks.
- Psychological Effects: When people discover they’ve been using unsafe products, they may lose trust not only in that brand but in sanitizers as a whole. This can discourage proper hygiene practices, undermining public health campaigns.
- Economic Burden: Mislabeled products that cause harm may lead to medical costs, lawsuits, recalls, and wasted consumer spending.
- Public Health Spread: Ineffective sanitizers contribute to the spread of infectious diseases, particularly in environments that rely heavily on them, like schools, hospitals, and public transportation.
The collective risk highlights why accurate labeling is not just a technical requirement but a public health imperative.
How Consumers Can Identify and Avoid Mislabeled Sanitizers
While regulatory bodies play a major role, consumers also need to stay proactive. Here are some practical tips for spotting mislabeled sanitizers:
- Check Alcohol Content: Look for at least 60% ethanol or 70% isopropyl alcohol. Anything less is ineffective.
- Avoid Methanol: Ensure the product does not list methanol or wood alcohol.
- Look for Clear Warnings: Proper sanitizers will include flammability warnings and keep-away instructions for children.
- Verify Manufacturer Credentials: Research the company. Reliable brands are transparent and often provide testing data.
- Cross-Check Recalls: Regulatory agencies frequently publish lists of recalled sanitizers. Staying updated helps avoid unsafe products.
- Be Wary of Overstated Claims: Phrases like “kills 100% of germs” or “FDA approved” are red flags.
- Smell and Texture: Poorly made sanitizers may have unusual odors (chemical, fuel-like) or sticky residues—signs of poor quality.
By combining regulatory oversight with consumer vigilance, mislabeling risks can be significantly reduced.
FAQs
Q1: Why do some hand sanitizers claim “FDA approved” when the FDA doesn’t actually approve them?
Answer: This is one of the most common mislabeling tactics. The FDA regulates hand sanitizers as over-the-counter (OTC) drugs, meaning manufacturers must comply with its standards.
However, the FDA does not issue official “approval” for these products in the same way it does for prescription drugs. When a label says “FDA approved,” it is misleading consumers into believing the product has undergone a more rigorous approval process than it actually has.
Many companies use this claim as a marketing ploy to build consumer trust. Unfortunately, it creates a false sense of safety and can lead people to choose products that don’t necessarily meet the required standards.
The FDA has repeatedly warned companies against making these claims, and products with such misleading labels are often subject to recall or warning letters. Consumers should instead look for terms like “FDA registered” or verify that the product complies with official FDA guidance.
Q2: How dangerous is methanol in mislabeled sanitizers?
Answer: Methanol, also known as wood alcohol, is highly toxic. While it is sometimes used industrially as a solvent or antifreeze, it has no place in consumer sanitizers. When applied to the skin in small amounts, methanol may not immediately cause noticeable harm, but repeated exposure can lead to systemic absorption.
This can result in symptoms like headaches, dizziness, nausea, blurred vision, and in severe cases, seizures or organ damage.
The greatest danger arises when methanol-containing sanitizers are ingested—whether accidentally by children or intentionally by individuals seeking alcohol substitutes. Even small amounts can cause blindness or death.
Because of these risks, regulatory agencies worldwide have banned methanol in consumer sanitizers. Yet, during times of high demand, some unscrupulous manufacturers have used it because it is cheaper than ethanol or isopropanol. This makes consumer vigilance critical, as mislabeled products may not always disclose the presence of methanol.
Q3: Can using a mislabeled hand sanitizer contribute to antibiotic resistance or “superbugs”?
Answer: Indirectly, yes. While hand sanitizers don’t contain antibiotics, using an ineffective or mislabeled sanitizer can contribute to the spread of germs. When people rely on these products thinking they are protected, pathogens continue to circulate in the community. Over time, this uncontrolled spread can increase the risk of infections that may require antibiotic treatment.
The overuse and misuse of antimicrobials—whether in healthcare, agriculture, or personal hygiene—are key drivers of antibiotic resistance. Though hand sanitizers themselves don’t create resistance, their mislabeling undermines infection control strategies.
This highlights why accurate labeling and effective formulations are critical for public health, especially in the fight against superbugs.
Q4: Are “natural” or “organic” hand sanitizers safer and more reliable?
Answer: Not necessarily. Many products labeled as “natural” or “organic” use this terminology as part of a marketing strategy, often without proper certification. While some plant-based sanitizers may include beneficial ingredients like aloe vera or essential oils, these do not replace the need for effective alcohol content.
The term “chemical-free” is especially misleading, since all substances are made of chemicals. What matters most is whether the product contains the right concentration of alcohol and is free from harmful ingredients like methanol.
Consumers should not assume that “natural” equals safe or effective. Instead, they should prioritize evidence-based factors such as proper alcohol levels, regulatory compliance, and transparent labeling.
Q5: How can I report a mislabeled or unsafe hand sanitizer?
Answer: If you suspect a hand sanitizer is mislabeled or unsafe, reporting it helps protect others. In the U.S., consumers can report issues directly to the FDA’s MedWatch program. Other countries have similar consumer safety hotlines or online reporting systems through health agencies.
When reporting, include as much detail as possible: the product name, manufacturer, lot number, place of purchase, and the specific issue observed (e.g., false alcohol percentage, missing warnings, suspicious smell). Regulatory agencies investigate these reports, and if necessary, issue recalls or warnings to protect the public.
By taking the step to report unsafe products, consumers actively contribute to public health and safety, ensuring that other buyers do not fall victim to mislabeled or dangerous sanitizers.
Conclusion
Hand sanitizers are meant to safeguard health, but mislabeling issues compromise their effectiveness and safety. Common problems such as incorrect alcohol content, hidden toxic ingredients, misleading claims, missing warnings, and false certifications highlight the need for stricter oversight and consumer awareness.
By understanding these mislabeling issues, recognizing red flags, and staying informed about recalls and regulations, consumers can make safer choices. Manufacturers, meanwhile, must prioritize transparency and compliance, recognizing that accurate labeling is not just a legal requirement but a moral obligation.
In the end, tackling mislabeling in hand sanitizers requires a combined effort from regulators, manufacturers, and consumers alike. Only through vigilance and accountability can we ensure that these essential hygiene tools truly deliver on their promise of protecting public health.