
By myhandsanitizershop January 7, 2026
Hand sanitizer sits at the intersection of chemistry, public health, and everyday convenience. Most people buy a bottle, glance at the label, and assume it “kills germs.”
But whether a product actually works—and whether it’s appropriate for your skin, your household, or your workplace—depends heavily on the active ingredients in hand sanitizers and how they’re formulated.
This guide explains what active ingredients in hand sanitizers are, how they work, what concentrations matter, and how to read labels like a pro. It also covers safety pitfalls (including contamination and recalls), special situations (kids, sensitive skin, food handling), and what the next few years may look like for sanitizer innovation and regulation.
A key takeaway from public health guidance is that soap and water remain the top choice in many situations, but alcohol-based sanitizer with at least 60% alcohol is a strong alternative when a sink isn’t available.
What “Active Ingredients in Hand Sanitizers” Actually Means
When you read a label, “active ingredient” isn’t marketing language—it’s a regulated term used for products that make antiseptic or antimicrobial claims. In simple terms, active ingredients in hand sanitizers are the specific chemicals that do the germ-killing work. Everything else in the bottle supports that work by improving feel, stability, shelf life, fragrance, or skin comfort.
This distinction matters because many people confuse “natural,” “plant-based,” or “essential-oil” claims with effectiveness. Those claims typically relate to inactive ingredients (like aloe, glycerin, or fragrances), not the proven germ-fighting part.
If a product claims it sanitizes or reduces germs, the active ingredients in hand sanitizers should be clearly listed with a percentage. In the retail market, the most common active ingredients in hand sanitizers fall into two broad categories:
- Alcohol-based actives (most widely recommended for rapid broad effectiveness)
- Non-alcohol actives (less common; relies on different chemistry and can be situation-dependent)
Another reason this definition matters: the label’s “Drug Facts” panel (when present) is a clue that you’re dealing with an over-the-counter antiseptic product, and the listed active ingredients in hand sanitizers should align with what regulators recognize for consumer rubs.
Finally, active ingredients in hand sanitizers don’t work in isolation. Concentration, evaporation rate, product volume used, and how long the hands stay wet all influence results. That’s why two products with the “same” active ingredient can perform differently in real life—especially if one dries instantly and the other stays wet long enough to do its job.
The Three Main Active Ingredients You’ll See Most Often

In consumer hand rub products, three active ingredients in hand sanitizers dominate label after label: ethyl alcohol (ethanol), isopropyl alcohol, and benzalkonium chloride.
Public regulatory communication also emphasizes that other active ingredients marketed for consumer hand sanitizer use are generally not considered legally marketed for that purpose, and consumers are advised to avoid them.
These three activities aren’t identical. They differ in how fast they work, how they feel on skin, what kinds of organisms they affect best, and how they behave when hands are visibly dirty or greasy.
Ethanol as an active ingredient in hand sanitizers
Ethanol (sometimes written as “ethyl alcohol”) is widely used because it works quickly and has strong activity against many common germs. Ethanol-based formulas are also common in settings where quick drying is important, like offices, schools, and retail.
When you see ethanol listed among the active ingredients in hand sanitizers, pay attention to the percentage. For everyday use, guidance commonly highlights at least 60% alcohol in the product for effectiveness.
Ethanol works primarily by disrupting proteins and damaging lipid membranes. That “membrane disruption” is one reason alcohol-based sanitizers tend to perform well against many enveloped viruses and a broad range of bacteria when used correctly. In practice, ethanol is often chosen for a balance of effectiveness, availability, and consumer acceptance.
Isopropyl alcohol as an active ingredient in hand sanitizers
Isopropyl alcohol is another mainstay. You’ll often see it as “isopropanol” or “2-propanol.” It behaves similarly to ethanol in many day-to-day scenarios, but the feel on skin and evaporation can differ.
If your skin reacts poorly to one alcohol type, a different formula may feel better—even when both list comparable active ingredients in hand sanitizers.
Like ethanol, isopropyl alcohol needs an adequate concentration and proper use to be dependable. A quick dab that dries in seconds isn’t the same as applying enough product to keep hands wet for proper rubbing time.
Benzalkonium chloride as an active ingredient in hand sanitizers
Benzalkonium chloride (often shortened to “BZK”) is a non-alcohol active ingredient in hand sanitizers. It’s used in some alcohol-free products. Benzalkonium chloride works differently from alcohol: it’s a quaternary ammonium compound that disrupts cell membranes in certain microbes.
Alcohol-free sanitizer can be appealing for people who dislike alcohol smell, have certain workplace restrictions, or want a less drying feel. But performance can vary by organism and use case, and product selection becomes more important. That’s why understanding active ingredients in hand sanitizers—and not just the brand—matters.
Why Alcohol Percentage Matters More Than Most People Think
When people ask whether a sanitizer “works,” they often focus on brand reputation or scent. But with alcohol-based products, the percentage is foundational. Guidance for community use commonly points to selecting products with at least 60% alcohol when soap and water aren’t available.
Here’s why the percentage matters:
Alcohol kills germs by denaturing proteins and disrupting membranes, but it requires a workable “contact window.” Too low a percentage may fail to inactivate microbes reliably.
Too high can sometimes evaporate so quickly that users under-rub, and certain formulations may feel harsher—though performance depends on the overall formula. What matters for real-world use is selecting a properly formulated product and using enough of it.
Another hidden issue: the number on the label might be listed as v/v (volume/volume) or w/w (weight/weight) depending on the product and manufacturer labeling practices.
Most consumers don’t need to calculate conversions, but you should understand that “60% alcohol” is a minimum guidance benchmark and not a magic shield. Product quality and correct technique still matter.
Alcohol percentage also interacts with inactive ingredients. If a product is overloaded with thickeners, heavy fragrances, or oils, it might feel nice but spread poorly, dry unevenly, or encourage too-small doses.
The active ingredients in hand sanitizers can only do their job if the sanitizer is applied adequately and rubbed thoroughly.
A practical rule: choose sanitizer with clearly labeled alcohol percentage that meets minimum guidance, and avoid products with vague labeling. If you cannot confirm the active ingredients in hand sanitizers and their concentration, you’re guessing—and in hygiene, guessing is rarely worth it.
How Active Ingredients in Hand Sanitizers Kill Germs (The Science, Made Simple)
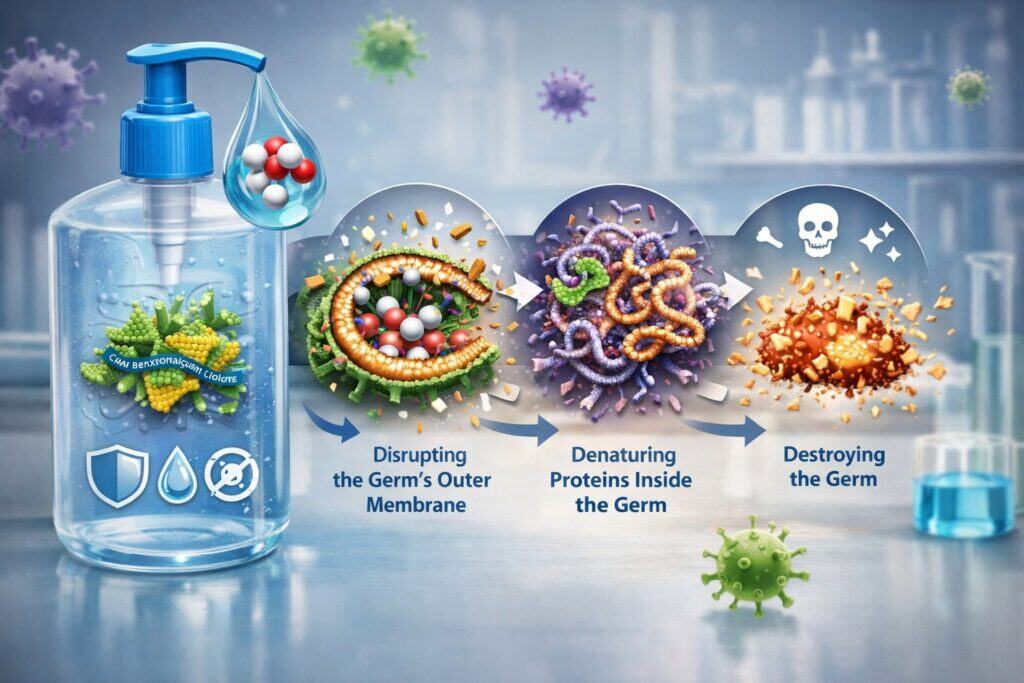
Understanding the mechanism helps you use sanitizer better and set realistic expectations.
Alcohol-based mechanisms: fast, broad, but technique-dependent
Alcohol-based active ingredients in hand sanitizers—ethanol and isopropyl alcohol—work quickly. They disrupt proteins and, for many organisms, damage the outer structures needed for survival. That’s why alcohol-based sanitizer is often the “grab-and-go” solution for everyday errands, shared surfaces, and public spaces.
But alcohol’s speed is a double-edged sword. Because it evaporates, the sanitizer must be used in sufficient quantity, and the hands must be rubbed to cover all surfaces before drying. Healthcare hygiene guidance notes that effectiveness depends on the volume applied.
If you apply too little, you may sanitize fingertips and miss thumbs, nail beds, and the backs of hands. If you stop rubbing early, you shorten contact time. The active ingredients in hand sanitizers can’t work well on areas they never reach.
Benzalkonium chloride mechanisms: different chemistry, different strengths
Benzalkonium chloride (BZK) behaves differently. It can persist longer on surfaces than alcohol in some formulations, and it often feels less drying. However, the spectrum of activity can be different across microbes and contexts, and performance depends on how the formula is designed and what organisms are present.
The big takeaway: different active ingredients in hand sanitizers have different “best use” scenarios. Alcohol-based products are often preferred when you want quick, broad action and you can tolerate the feel.
Benzalkonium chloride products may be chosen for comfort or specific settings, but selection should be careful and aligned with labeling and guidance.
The role of friction and coverage
Even with the best active ingredients in hand sanitizers, friction matters. Rubbing helps distribute product, dislodge dirt films, and move sanitizer into creases and around nails. That’s why technique is part of the “ingredient story.” The chemistry needs the choreography.
Alcohol-Based vs Alcohol-Free: Choosing What Fits Your Real Life
The “best” sanitizer isn’t universal. It depends on where you use it, how often, and what you’re trying to reduce risk from.
When alcohol-based active ingredients in hand sanitizers are a strong choice
Alcohol-based sanitizer is often ideal for:
- Public outings where sinks aren’t available
- Commuting, shopping carts, and shared door handles
- Quick hygiene after touching commonly handled surfaces
- Office environments where speed matters
Public guidance commonly emphasizes alcohol-based sanitizer (at appropriate concentration) when soap and water aren’t available.
Alcohol-based active ingredients in hand sanitizers are also easy to evaluate on labels because they list a clear percent. That transparency makes it easier for consumers to compare products.
When alcohol-free active ingredients in hand sanitizers may be preferred
Alcohol-free sanitizer products (often using benzalkonium chloride) can be useful for:
- People with strong alcohol sensitivity or aversion to alcohol odor
- Situations where flammability is a major operational concern (while still following local workplace rules)
- Users who need gentler-feeling products for frequent daily use
That said, consumers should be wary of products claiming sanitizer benefits while using unfamiliar activities. Regulatory messaging advises avoiding hand sanitizers marketed with active ingredients outside of ethanol, isopropyl alcohol, or benzalkonium chloride.
The “soap and water first” reality
Even the best active ingredients in hand sanitizers don’t replace handwashing in every scenario. Soap and water physically remove many types of germs and also help remove chemicals and grime. Public guidance continues to highlight that handwashing is the best way to get rid of germs in most situations.
So the smartest approach is not “sanitizer vs soap,” but “right tool, right time,” guided by active ingredients in hand sanitizers and real-world conditions.
How to Read a Label Like a Pro (Drug Facts, Concentration, and Red Flags)
If you want to shop intelligently, labels are your best friend. The key is knowing what information matters most.
Start with the active ingredients in hand sanitizers section
Look for:
- The named active ingredient (ethanol, isopropyl alcohol, or benzalkonium chloride)
- The concentration (percentage)
- Clear directions and warnings
If the label doesn’t clearly state the active ingredients in hand sanitizers and their concentration, treat it as a red flag. “Kills 99.9% of germs” is not a substitute for transparent active ingredient labeling.
Check directions: they reveal whether the formula expects proper use
Legit products typically instruct you to:
- Apply a certain amount (often “enough to cover hands”)
- Rub hands together until dry
- Avoid eyes and ingestion
- Keep out of reach of children
Those instructions matter because they align with how active ingredients in hand sanitizers need contact time and coverage to work.
Red flags that often signal low value or higher risk
Be cautious of:
- Vague ingredient claims (“plant-based sanitizer” with no listed antiseptic active)
- Overly perfumed products that irritate skin and discourage thorough rubbing
- Products with a strong chemical odor that doesn’t resemble typical ethanol/isopropyl profiles
- Labels that look unofficial or lack standard formatting for warnings and directions
Also pay attention to updates related to recalls and unsafe products. FDA has maintained consumer-facing information and directs people to recall resources for sanitizer products, including those affected by contamination issues.
Reading labels isn’t about being paranoid—it’s about ensuring the active ingredients in hand sanitizers are present, properly concentrated, and paired with directions that match real effectiveness.
Safety Risks: Methanol, Contamination, and Why “Cheap” Can Get Expensive
Most people worry about germs, but the bigger risk sometimes comes from the product itself—especially when supply chains are stressed or you buy from unknown sources.
Methanol contamination and toxic alcohols
One of the most serious safety concerns in the sanitizer world has been contamination with methanol, which is toxic if absorbed through skin, inhaled in significant amounts, or ingested. FDA communications and recall notices have repeatedly addressed this risk and have tied specific recalls to methanol presence in some products.
The practical safety rule is simple: only purchase sanitizer from reputable retailers and brands that provide clear labeling. If a sanitizer smells unusually harsh, causes immediate burning, or lacks clear active ingredient disclosure, stop using it.
Microbial contamination and manufacturing quality
Not all sanitizer risks are about alcohol purity. Some recalls have involved microbial contamination in topical products, which can be dangerous especially for people with weakened immune systems or when applied to compromised skin.
That’s why “active ingredients in hand sanitizers” is only half the story. Manufacturing quality, packaging integrity, and storage conditions are the other half.
What to do if you suspect a product is unsafe
If you’re worried about a specific product:
- Stop using it immediately
- Check whether it appears in recent recall listings or safety alerts
- Do not attempt to “fix” it by mixing or adding alcohol (this is not recommended and may not create an effective product)
The safest move is disposal according to local guidance and replacing with a reputable, clearly labeled product.
Skin Health and Frequent Use: Balancing Germ Control with a Healthy Barrier
Frequent sanitizer use can be tough on skin, even when the active ingredients in hand sanitizers are appropriate and effective. Dryness, cracking, and irritation aren’t just uncomfortable—they can undermine hygiene by making people avoid hand cleaning or by creating damaged skin that feels painful.
Why alcohol-based actives can dry skin
Alcohol evaporates and can strip oils from the skin surface. Many formulas include moisturizers like glycerin to help counteract dryness, but your personal experience will vary depending on the formula, how often you apply it, and your baseline skin type.
Healthcare guidance notes that alcohol-based hand rubs can be associated with improved skin condition compared with frequent soap-and-water washing in some settings, but technique and product choice still matter.
The role of inactive ingredients in comfort
Inactive ingredients are not the germ killers, but they can make or break the user experience. Look for:
- Humectants (like glycerin) to reduce dryness
- Minimal fragrance if you’re sensitive
- Formulas that don’t leave sticky residue (which can encourage underuse)
The best “skin-friendly” product is the one you’ll use correctly and consistently—without irritation forcing you to stop.
A practical routine for frequent sanitizer users
If you sanitize many times a day:
- Use alcohol-based sanitizer with suitable concentration and rub thoroughly
- Moisturize regularly (especially after washing hands with soap)
- If your skin cracks or bleeds, prioritize gentle soap-and-water when available and consult a clinician if severe
Skin comfort supports compliance, and compliance is what makes active ingredients in hand sanitizers useful in the real world.
Special Situations: Food Handling, Schools, Travel, and Healthcare Settings
Your environment changes what “best” means.
Food handling and greasy hands
If your hands are visibly dirty or greasy, sanitizer may struggle to reach microbes through the film. In these cases, soap and water is the better first step. Public guidance repeats that handwashing is best in many situations.
Schools and child safety
In schools and at home with kids:
- Choose products with clearly labeled active ingredients in hand sanitizers
- Store bottles out of reach of very young children
- Supervise use to prevent accidental ingestion
Kids often use too little or wipe hands before drying, which reduces effectiveness. Teaching them to rub until dry is as important as the active ingredient itself.
Travel and public spaces
For travel, especially air travel or road trips, alcohol-based sanitizer is practical because it’s portable and fast. Choose a leak-proof container and avoid leaving it in extreme heat (like a hot car), which can degrade product quality over time.
Healthcare and high-risk environments
In healthcare environments, technique and volume matter intensely. Guidance for clinical settings emphasizes that efficacy depends on applying the right volume.
In these environments, the active ingredients in hand sanitizers must be paired with strict protocols, and soap-and-water remains essential in specific pathogen scenarios.
Common Myths About Active Ingredients in Hand Sanitizers (And What’s Actually True)
Misinformation spreads faster than germs. Clearing up myths helps you buy and use sanitizer more effectively.
Myth: “If it stings, it’s working”
Stinging can mean you have cracked skin or irritation from fragrance or other inactive ingredients. It doesn’t prove the active ingredients in hand sanitizers are present at an effective concentration. Some perfectly effective products won’t sting at all.
Myth: “Natural oils are the same as sanitizer”
Essential oils may smell pleasant, but they are not a replacement for recognized active ingredients in hand sanitizers. If the label doesn’t list a proven active ingredient and a concentration, the product should not be trusted for sanitizing claims.
Myth: “More alcohol is always better”
The best product is the one that balances effective concentration with usability. If a product dries too fast or feels too harsh, people often use too little or rush. That user behavior defeats the purpose of having strong active ingredients in hand sanitizers.
Myth: “I can make my own and it’s the same”
Regulators have warned against homemade hand sanitizer due to risks of incorrect formulation and ineffectiveness, and also because mixing or adding alcohol to other products doesn’t reliably create an effective sanitizer.
Truth: buying a reputable product with clear active ingredient labeling is safer and more reliable.
Storage, Shelf Life, and Performance Over Time
Even the right active ingredients in hand sanitizers won’t perform well if the product is stored badly or used past its effective life.
Does hand sanitizer expire?
Yes, many products include an expiration date. Over time, alcohol can evaporate—especially if caps are left loose—reducing the effective concentration. Heat can also degrade some inactive ingredients and change texture.
Best storage practices
- Keep containers tightly closed
- Store at room temperature where possible
- Avoid long-term storage in hot cars or direct sun
- Replace bottles that leak, separate, or smell “off”
Performance clues to watch
If a sanitizer suddenly becomes watery, lumpy, unusually sticky, or smells drastically different, it may have changed composition. The active ingredients in hand sanitizers might no longer be at the intended concentration, or the formula may have degraded.
In workplaces that stored large volumes during supply surges, safe handling and management guidance has even been issued at local levels due to the flammability and volume risks of alcohol-based sanitizer stockpiles.
Future Prediction: Where Hand Sanitizer Active Ingredients and Formulas Are Headed
The next wave of sanitizer innovation is likely to focus on three goals: better skin tolerance, stronger real-world reliability, and clearer compliance expectations.
Likely trends in the next few years
- Skin-barrier friendly formulations: Expect more products pairing alcohol-based active ingredients in hand sanitizers with improved moisturizers, barrier-support ingredients, and less irritating fragrances. The winning formulas will reduce dryness without reducing performance.
- Improved packaging and dosing: “How much is enough?” is a recurring problem. Future packaging may push metered pumps, foams that spread evenly, and labels that better communicate correct dosing—because the active ingredients in hand sanitizers depend on adequate volume and coverage.
- More transparency and faster recall communication: Consumers have learned to look for safety alerts and recalls.
We can expect continued pressure for clearer labeling, better traceability, and faster public communication when contamination occurs. FDA has continued to direct consumers to recall databases and related resources for sanitizer safety updates. - Potential shifts in regulatory evaluations: Regulatory evaluation of consumer antiseptic rub active ingredients has included deferred determinations for certain activities in past rulemaking, reflecting ongoing data needs and evolving standards.
While consumers don’t need to follow every update, it’s a reminder to stick with recognized active ingredients in hand sanitizers and reputable brands.
What probably won’t change
Alcohol-based sanitizer with appropriate concentration will likely remain a central option for rapid hand hygiene when sinks aren’t available, because it’s practical, fast, and well understood. The “future” is less about replacing alcohol and more about making the overall experience safer, gentler, and more foolproof.
FAQs
Q.1: What are the most effective active ingredients in hand sanitizers?
Answer: For everyday consumer use, the most common active ingredients in hand sanitizers are ethanol (ethyl alcohol) and isopropyl alcohol, typically at concentrations that meet minimum effectiveness guidance. Public guidance commonly recommends choosing sanitizer with at least 60% alcohol when soap and water aren’t available.
A third active ingredient seen in some products is benzalkonium chloride, which is alcohol-free and works differently. The best choice depends on your skin tolerance, setting, and the product’s label clarity.
Q.2: Is benzalkonium chloride as good as alcohol?
Answer: Benzalkonium chloride can be useful, but it isn’t identical to alcohol-based active ingredients in hand sanitizers. It has a different spectrum of activity and can be more formula-dependent. If you choose it, prioritize reputable brands, clear labeling, and proper use directions.
Also be cautious of products with unfamiliar actives; FDA consumer guidance has emphasized avoiding hand sanitizers marketed with active ingredients outside ethanol, isopropyl alcohol, or benzalkonium chloride.
Q.3: Why does my sanitizer say 70% or 62%—does it matter?
Answer: Yes. The percentage tells you how much of the active ingredients in hand sanitizers is present. Guidance commonly highlights a minimum threshold (like at least 60% alcohol) for effectiveness in community settings.
Beyond minimums, how you apply it—enough volume, full coverage, rub until dry—makes a huge difference.
Q.4: Can I add alcohol to a lotion or a low-alcohol sanitizer to “upgrade” it?
Answer: This is not recommended. FDA consumer guidance notes that adding alcohol to an existing non-alcohol sanitizer is unlikely to result in an effective product, and it also discourages making your own hand sanitizer. If you need effective protection, buy a properly formulated product with clear active ingredient labeling.
Q.5: How much hand sanitizer should I use?
Answer: Use enough to cover all hand surfaces and rub until dry. In clinical guidance, effectiveness is linked to the volume applied, which supports the idea that “a little dab” may be insufficient.
A good rule is: palms, backs of hands, between fingers, thumbs, fingertips, and around nails should all feel wet during rubbing.
Q.6: What should I do if I hear about a recall?
Answer: Stop using the product, check official recall listings, and dispose of it according to local guidance. FDA has directed consumers to recall resources and databases for sanitizer safety information, including issues like contamination.
If you experience symptoms after using a recalled topical product—especially on broken skin—contact a healthcare provider.
Conclusion
Choosing a hand sanitizer shouldn’t feel like guesswork. The simplest way to make a smart decision is to focus on the active ingredients in hand sanitizers first, then evaluate the formula, labeling, and safety signals.
For most daily situations when a sink isn’t available, alcohol-based sanitizer (ethanol or isopropyl alcohol) with adequate concentration is a reliable choice, supported by public guidance that commonly recommends at least 60% alcohol.
But effectiveness isn’t just chemistry—it’s also behavior. Use enough product, rub thoroughly, and don’t cut the process short.
At the same time, safety and quality matter. Contamination risks, recalls, and unclear labeling are real concerns. Stick with reputable brands, avoid products with unfamiliar active ingredients, and don’t try to “DIY” effectiveness by mixing formulas.
Looking ahead, the future of active ingredients in hand sanitizers will likely emphasize better skin tolerance, clearer dosing, and stronger transparency. The best sanitizer is the one that combines the right active ingredient, a trustworthy label, and a formula you’ll actually use correctly—every time your hands need it.